Master the CSCA Exam: Preparation Course & Test Coaching
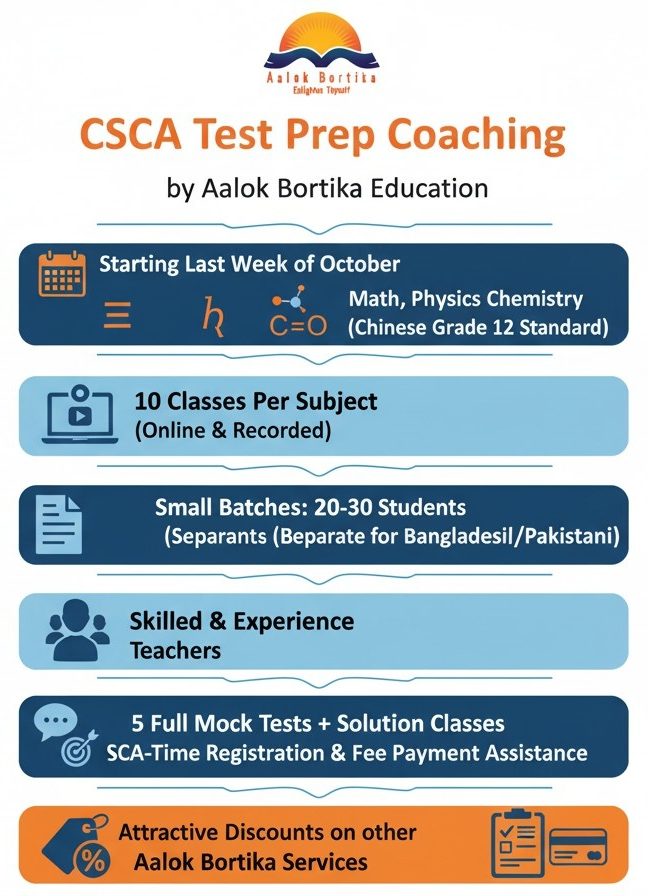
Aalok Bortika Education is thrilled to launch our highly anticipated CSCA Test Preparation Coaching and CSCA Mock Test Series program, starting the last week of October! If you’re aiming for a successful admission or require passing this crucial Study in China Admission Test for a China CSC Scholarship Exam, our comprehensive course is your essential roadmap to success.
Why Our CSCA Preparation Program is the Best Choice: The CSCA exam is a uniquely challenging entrance requirement, particularly for science subjects like Mathematics, Physics, and Chemistry, as it adheres to the demanding Chinese Senior High School (Grade 12) standard. Generic materials won’t suffice. That’s why our subject-matter experts have performed rigorous analysis of previous years’ university question papers to craft a full-fledged, comprehensive module designed for the exact format and difficulty of the exam. This preparation is directly aligned with the official CSCA exam syllabus.
What’s Inside Your CSCA Success Package?
We’ve structured our program to ensure maximum success and flexibility, catering specifically to the needs of South Asian students.
- Targeted Subject Prep: Get 10 dedicated classes for each core subject—comprehensive CSCA Math Physics Chemistry Prep.
- Online Convenience: All classes are conducted online, and recorded classes are provided on your student dashboard for review anytime, anywhere.
- Exclusive Batches: Benefit from personalized attention in small batches (20-30 students). We offer separate, tailored batches for the CSCA exam preparation course for any country if there is enough students to fill batches with that country students. Initially we are arranging exclusive batches for Bangladeshi students, Moroccan Students and Pakistani students.
- Expert Instruction: Learn from a panel of skilled and highly experienced teachers who specialize in the Chinese Senior High School Exam Prep standard.
- Mock Test Mastery: Solidify your knowledge with 5 full Mock Tests after the lectures, followed by dedicated solution classes for in-depth performance analysis.
- Dedicated Support: Receive continuous support through our all-time assistance group for instant query resolution.
- Registration Made Easy: We provide complete assistance with your online registration and fee payment for the actual CSCA Test.
- Exclusive Perks: All participants will receive attractive discounts on other valuable service packages offered by Aalok Bortika Education.
Your dream of studying in China is within reach. Let Aalok Bortika Education be the light that “Enlightens Thyself” and guides you to success!
Enrollment has officially opened! Secure your spot in the CSCA Test Preparation Coaching batch built for your success today.


To know Course Outline of our Course click the tabs below
CSCA Mathematics Exam Preparation Course Outline
Lecture Sheet 1: Foundations – Sets & Logic
- Topics Covered:
- Definition, representation (roster, set builder), and properties of sets.
- Relationships between sets: subsets, proper subsets, and the empty set.
- Set operations: union ($A \cup B$), intersection ($A \cap B$), and complement ($C_U A$)3
Lecture Sheet 2: Inequalities
- Topics Covered:
- Basic properties and axioms of inequalities.
- Solution methods for quadratic inequalities (e.g., $ax^2 + bx + c > 0$).
- Solution methods for rational inequalities (e.g., $\frac{P(x)}{Q(x)} \ge 0$).
Lecture Sheet 3: Core Concepts of Functions
- Topics Covered:
- Definition of a function, domain, and range.
- Properties of functions: monotonicity (increasing/decreasing) and parity (even/odd functions).
- Graphical representation of functions and transformations (shifting, stretching).
Lecture Sheet 4: Elementary Functions
- Topics Covered:
- Power functions ($y=x^a$).
- Exponential functions ($y=a^x$) and logarithmic functions ($y=\log_a x$).
- Trigonometric functions (sine, cosine, tangent), their graphs, and basic identities.
Lecture Sheet 5: Sequences & Introduction to Calculus
- Topics Covered:
- Arithmetic sequences: general term formula ($a_n$) and summation ($S_n$).
- Geometric sequences: general term formula and summation.
- Introduction to derivatives: definition and geometric meaning (slope of a tangent).
- Simple applications of derivatives, such as finding the monotonicity of a function.
Lecture Sheet 6: Plane Analytic Geometry I (Lines & Circles)
- Topics Covered:
- Equations of lines (slope-intercept, point-slope, general form).
- Relationships between lines (parallel, perpendicular).
- Standard and general equations of a circle.
- Positional relationship between lines and circles (tangent, secant).
- Gaokao Context: Analytic geometry is a major component of the exam. Problems often combine algebraic manipulation with geometric intuition.
Lecture Sheet 7: Plane Analytic Geometry II (Conic Sections)
- Topics Covered:
- Definitions and standard equations of the ellipse, hyperbola, and parabola18.
- Key properties: foci, vertices, eccentricity, and asymptotes (for hyperbolas)19.
Lecture Sheet 8: Vectors, Complex Numbers & Solid Geometry
- Topics Covered:
- Vector concepts and operations (addition, subtraction, scalar multiplication).
- Basic concepts and operations of complex numbers (addition, subtraction, multiplication).
- Rectangular coordinate system in 3D space.
- Properties of simple geometric solids (cubes, prisms, pyramids).
Lecture Sheet 9: Probability & Statistics
- Topics Covered:
- The classical probability model and methods for calculating probability.
- Numerical characteristics of data: mean and variance.
- Basic understanding of the normal distribution and its properties.
Lecture Sheet 10: Final Review & Exam Strategy
- Topics Covered:
- Comprehensive review of all content modules.
- Timed practice drills focusing on the exam format: 48 multiple-choice questions in 60 minutes.
- Strategies for single-answer multiple-choice questions: elimination, substitution, and special value testing.
- Time management to ensure all questions are attempted.
CSCA Physics Preparatory Course: 10-Lecture Outline
This 10-lecture course is designed to provide a comprehensive review of the CSCA Physics Syllabus (2025) with a preparation intensity inspired by the Gaokao. The primary goal is to build deep conceptual mastery, rapid problem-recognition, and strategic test-taking skills for a high-pressure, multiple-choice environment.
Module 1: Mechanics (Lectures 1-3)
Lecture 1: Foundations of Motion (Kinematics & Newton’s Laws)
- Core Concepts:
- Kinematics: Displacement, velocity (average vs. instantaneous), acceleration.
- Uniform acceleration motion: Key equations, graphical analysis ($v-t$, $x-t$ graphs).
- Free-fall motion.
- Newton’s laws of motion: First, Second ($F=ma$), and Third laws.
- Application: Free-body diagrams, static and kinetic friction, forces on inclines, connected objects (e.g., pulley systems).
- CSCA Focus:
- Mastery of free-body diagrams.
- Rapidly interpreting kinematic graphs.
- Identifying the correct frame of reference and system to analyze.
Lecture 2: The Conservation Laws (Work, Energy & Momentum)
- Core Concepts:
- Work and power (constant and variable forces).
- Work-Energy Theorem.
- Kinetic Energy ($KE$) and Potential Energy ($PE$ – gravitational and elastic).
- Law of Conservation of Mechanical Energy.
- Impulse and Momentum ($p=mv$).
- Impulse-Momentum Theorem.
- Law of Conservation of Momentum (1D and 2D collisions).
- CSCA Focus:
- Quickly determining if energy, momentum, or both are conserved.
- Problem-solving using “before and after” state analysis.
- Applying conservation laws vs. $F=ma$ (knowing which is faster).
Lecture 3: Rotational & Oscillatory Motion (Circular, Gravitation & SHM)
- Core Concepts:
- Uniform circular motion: Centripetal acceleration and centripetal force.
- Applications: Banked curves, objects in vertical circles.
- Newton’s law of universal gravitation ($F=G \frac{m_1 m_2}{r^2}$).
- Gravitational force as centripetal force (orbits).
- Simple Harmonic Motion (SHM): Key characteristics, period, frequency.
- Mechanical waves: Transverse vs. longitudinal, wavelength, frequency, wave speed.
- CSCA Focus:
- Identifying the source of centripetal force in various scenarios.
- Distinguishing SHM from other oscillatory motion.
- Understanding the relationship between gravitation and satellite/planetary motion.
Module 2: Electromagnetism (Lectures 4-6)
Lecture 4: Static Charges & Steady Currents (Electrostatics & DC Circuits)
- Core Concepts:
- Electrostatics: Coulomb’s Law, electric field strength ($E$), electric potential ($V$).
- Relationship between $E$-field and electric potential.
- Direct Current (DC) Circuits: Ohm’s Law ($V=IR$).
- Series and parallel circuits: Calculating equivalent resistance.
- Electric work ($W=qU$) and power ($P=IV$).
- CSCA Focus:
- Superposition principle for $E$-fields and potentials.
- Rapidly simplifying complex resistor networks (series/parallel).
- Analyzing changes in circuit (e.g., “what happens to the brightness of bulb A if bulb B burns out?”).
Lecture 5: Moving Charges & Magnetic Fields (Magnetism)
- Core Concepts:
- Magnetic field (magnetic induction, $B$).
- Magnetic force on a current-carrying wire (Ampere’s force: $F=ILB$).
- Magnetic force on a moving charge (Lorentz force: $F=qvB$).
- Right-hand rules (for force direction, field from current).
- Application: Charged particle motion in a uniform magnetic field (circular path).
- CSCA Focus:
- Mastery of all right-hand rules; 3D visualization.
- Combining electric and magnetic fields (e.g., velocity selectors).
- Deriving radius and period for charged particles in $B$-fields.
Lecture 6: Dynamic Fields (Electromagnetic Induction)
- Core Concepts:
- Magnetic flux ($\Phi$).
- Faraday’s Law of Induction: Induced electromotive force (EMF).
- Lenz’s Law: Determining the direction of induced current.
- Applications: Motional EMF ($E=BLv$), generators.
- CSCA Focus:
- Lenz’s Law is critical: “opposing the change in flux.”
- Interpreting graphs of $\Phi$ vs. $t$ to find induced EMF.
- Problems combining induction with forces and energy.
Module 3: Thermodynamics, Optics & Modern Physics (Lectures 7-9)
Lecture 7: Thermodynamics
- Core Concepts:
- Kinetic theory of gases (microscopic origin of pressure and temperature).
- Ideal gas equation of state ($PV=nRT$).
- First Law of Thermodynamics ($\Delta U = Q + W$).
- Understanding $U$ (internal energy), $Q$ (heat), and $W$ (work).
- Isobaric, isochoric, isothermal, and adiabatic processes.
- CSCA Focus:
- Applying the First Law to different processes.
- Interpreting $P-V$ diagrams (calculating work, identifying processes).
- Connecting microscopic kinetic theory to macroscopic variables.
Lecture 8: Optics
- Core Concepts:
- Geometrical Optics: Law of reflection.
- Law of refraction (Snell’s Law: $n_1 \sin\theta_1 = n_2 \sin\theta_2$).
- Total internal reflection (TIR) and critical angle.
- Physical Optics: Young’s double-slit interference (path difference, fringe spacing).
- Single-slit diffraction.
- CSCA Focus:
- Rapidly applying Snell’s Law and TIR.
- Distinguishing interference and diffraction patterns and their cause (path difference).
- Understanding the conditions for constructive and destructive interference.
Lecture 9: Modern Physics
- Core Concepts:
- Photoelectric effect: Einstein’s equation ($E_k = hf – W$), threshold frequency, work function.
- Particle-wave duality (photons).
- Atomic structure: Bohr model for hydrogen (energy levels, quantization).
- Atomic transitions (emission and absorption of photons).
- Fundamentals of nuclear physics: Nuclear composition (protons, neutrons).
- Nuclear reactions: Alpha, Beta, Gamma decay; nuclear fission and fusion.
- Mass-energy equivalence ($E=mc^2$).
- CSCA Focus:
- Conceptual mastery: (e.g., intensity vs. frequency in the photoelectric effect).
- Calculating energy/wavelength of photons from atomic transitions.
- Balancing nuclear reaction equations and applying conservation laws.
Module 4: Final Synthesis (Lecture 10)
Lecture 10: Final Review & Timed Exam Strategy
- Core Concepts:
- Review of all 5 modules, focusing on high-yield formulas and concepts.
- Connecting concepts across modules (e.g., energy conservation in mechanics, E&M, and thermodynamics).
- CSCA Focus:
- Strategy for 48 Questions in 60 Minutes:
- Triage: Identifying “quick-win” questions vs. “time-sink” questions.
- Pacing: Maintaining an average of 1.25 minutes per question.
- “Process of elimination” for multiple-choice.
- Guessing strategies when stuck.
- Activity: Timed mini-exam (e.g., 24 questions in 30 minutes) followed by a detailed walkthrough of the solutions and strategies.
- Strategy for 48 Questions in 60 Minutes:
CSCA Chemistry Preparatory Course: 10-Lecture Outline
This course is designed to comprehensively cover the CSCA Chemistry Examination Syllabus (2025 Edition) with a focus on the problem-solving strategies and depth of understanding required in a Gaokao-style preparatory context.
Lecture 1: Core Concepts & The Mole
- Syllabus Module: Basic Chemical Concepts and Calculations
- Key Topics:
- Classification of matter (elements, compounds, mixtures) and states of matter.
- Chemical notation: Writing and interpreting chemical formulas and names.
- The mole: Mastering calculations involving the amount of substance (Avogadro’s constant, molar mass).
- Writing and balancing chemical equations (including net ionic equations).
- Stoichiometry: Mass-mole, mass-volume, and mole-mole calculations in chemical reactions.
- Focus Skill: High-speed stoichiometric calculations and unit conversions.
Lecture 2: Solutions and Gases
- Syllabus Module: Basic Chemical Concepts and Calculations
- Key Topics:
- Solutions: Understanding molarity, mass percentage, and dilution calculations.
- Introduction to electrolytes: Strong vs. weak electrolytes.
- Solution pH: Calculations involving pH, pOH, [H+], and [OH-] for strong acids and bases.
- Gases: Application of the Ideal Gas Law (PV=nRT) and its relationship to molar volume.
- Focus Skill: Quantitative problem-solving for solutions and gases.
Lecture 3: Atomic Structure & The Periodic Table
- Syllabus Module: Chemical Theories and Laws
- Key Topics:
- Atomic structure: Protons, neutrons, electrons, isotopes, and electron configuration (up to Z=36).
- The Periodic Law: Understanding periods, groups, and blocks (s, p, d).
- Periodic trends: Atomic radius, ionization energy, electronegativity, and metallic/non-metallic character.
- Positional relationships: Deducing element properties based on table position.
- Focus Skill: Predicting chemical and physical properties using periodic trends.
Lecture 4: Chemical Bonds & Intermolecular Forces
- Syllabus Module: Chemical Theories and Laws
- Key Topics:
- Chemical bonds: Ionic bonds, covalent bonds (polar and nonpolar), and metallic bonds.
- Lewis structures and simple molecular geometry (VSEPR for basic shapes like linear, trigonal planar, tetrahedral).
- Intermolecular forces: Hydrogen bonds, dipole-dipole forces, and London dispersion forces.
- Impact of bonding and IMFs on physical properties (melting point, boiling point, solubility).
- Focus Skill: Relating molecular structure to macroscopic properties.
Lecture 5: Inorganic Chemistry I: Non-Metals
- Syllabus Module: Properties and Reactions of Substances
- Key Topics:
- In-depth review of key non-metal elements: Halogens (Cl, Br, I), Oxygen (Ozone), Sulfur, Nitrogen, Phosphorus, Carbon, and Silicon.
- Properties of their common oxides (e.g., SO₂, SO₃, NO, NO₂, CO, CO₂, SiO₂) and corresponding acids (e.g., H₂SO₄, HNO₃, H₂CO₃, H₃PO₄).
- Reactions of common bases (e.g., ammonia).
- Focus Skill: Memorization and application of key non-metal reactions.
Lecture 6: Inorganic Chemistry II: Metals
- Syllabus Module: Properties and Reactions of Substances
- Key Topics:
- Properties of common metals: Alkali metals (Na), Alkaline earth metals (Mg, Ca), and key transition/main group metals (Al, Fe, Cu).
- Reactions with water, acids, and non-metals.
- Properties of their oxides and hydroxides (e.g., Al(OH)₃ amphoterism).
- Common salts: Properties and reactions of carbonates, sulfates, nitrates, and chlorides.
- Focus Skill: Identifying metals and their compounds’ characteristic reactions.
Lecture 7: Redox & Ionic Reactions
- Syllabus Module: Properties and Reactions of Substances / Chemical Theories and Laws
- Key Topics:
- Redox Reactions: Identifying oxidizing/reducing agents and assigning oxidation states.
- Balancing complex redox reactions (half-reaction method recommended).
- Theories of electrolyte solutions: Ionization and dissociation.
- Ionic Reactions: Writing and balancing net ionic equations.
- Ionic coexistence: Determining which ions can (and cannot) exist together in solution.
- Ionic testing methods: Qualitative analysis for common ions (e.g., Cl⁻, SO₄²⁻, CO₃²⁻, NH₄⁺, Fe³⁺, Cu²⁺).
- Focus Skill: Mastering net ionic and redox equation balancing.
Lecture 8: Chemical Kinetics & Equilibrium
- Syllabus Module: Chemical Theories and Laws
- Key Topics:
- Reaction Rate: Factors affecting rate (concentration, temperature, pressure, catalyst).
- Chemical Equilibrium: The concept of dynamic equilibrium and the equilibrium constant (Kc, Kp).
- Le Chatelier’s Principle: Predicting shifts in equilibrium due to changes in concentration, pressure, and temperature.
- Weak electrolyte equilibrium: Ionization of weak acids/bases (qualitative understanding).
- Focus Skill: Qualitative and quantitative analysis of equilibrium systems.
Lecture 9: Basic Organic Chemistry
- Syllabus Module: Properties and Reactions of Substances
- Key Topics:
- Hydrocarbons: Alkanes, alkenes (addition reactions), alkynes (addition reactions), and aromatic compounds (Benzene).
- Nomenclature of simple organic compounds.
- Isomerism (structural and cis-trans).
- Common derivatives: Alcohols (oxidation), aldehydes (oxidation), carboxylic acids (esterification), and esters (hydrolysis).
- Introduction to basic organic polymers (e.g., polyethylene, PVC).
- Focus Skill: Identifying functional groups and predicting their characteristic reactions.
Lecture 10: Chemical Experiments & Applications
- Syllabus Module: Chemical Experiments and Applications
- Key Topics:
- Laboratory safety rules and recognition of hazard symbols.
- Use of common laboratory apparatus (e.g., volumetric flask, burette, condenser).
- Methods for separation and purification of substances: Filtration, distillation, extraction, and chromatography.
- Preparation and identification of common gases (e.g., H₂, O₂, Cl₂, NH₃, CO₂).
- Analysis of industrial processes: Principles of ammonia synthesis (Haber-Bosch process) as an application of equilibrium.
- Focus Skill: Connecting experimental design to chemical principles.






